
Have you ever thought, “why there is a need for ISO 5832-3 standard while considering Titanium material for implant production??”
The ISO 5832-3 specification qualifies Ti6Al4V Titanium Grade for medical implant applications.
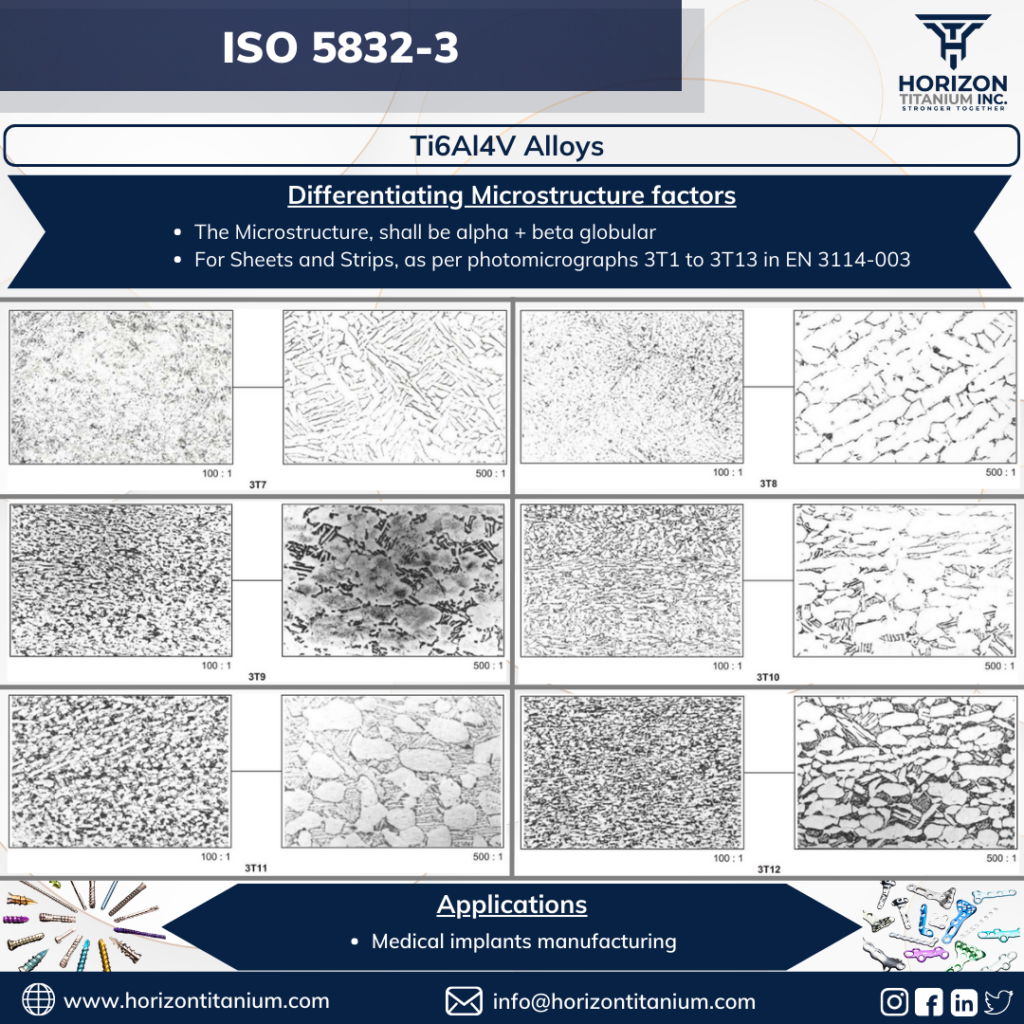
These specifications certify Ti6Al4V grade based on their microstructure morphologies according to ISO 20160 for round bars and EN 3114-003 for sheets.
ISO 20160 offers reference transverse section microstructure micrographs with designations ranging from A1 to A24 at 200x magnifications with varying morphologies of alpha and beta globular phase distribution for round bars. A1 is the best example of a fine globular alpha and beta phase distribution. As we can see from A1 to A9, the distribution and sizes of alpha and beta phases vary but are all considered acceptable because they are all in a globular shape, which is the most dominant shape for the hindrance of dislocation movements. As a result, the mechanical property of the Titanium improves.
EN 3114-003 offers reference transverse section microstructure micrographs with designations ranging from 3T1 to 3T38 at 100x and 500x magnifications for sheets/strips. The identical phenomena happen in the sheets, as explained above in the round bar section, although the morphologies of the alpha and beta phases differ minimally. Because 3T1 and 3T13 are globular morphologies with microstructures, they are suitable for medical implant manufacturing.
Are you an implant manufacturer and looking for the right Titanium material?
Are you an Orthopaedic surgeon and curious to know about Titanium?
Send us your queries at info@horizontitanium.com, and we will share the microstructure reports of our Titanium materials with you in advance for your reference.